Ground State Electron Configuration of Fe
Atoms may occupy different energy states. Fe has an atomic number of 26 which means that the atoms have 26 protons in their nuclei and 26 electrons in their electron clouds if they are neutral.

Electron Configuration For Iron Fe Fe2 And Fe3
An excited state is an energy level of an atom ion.
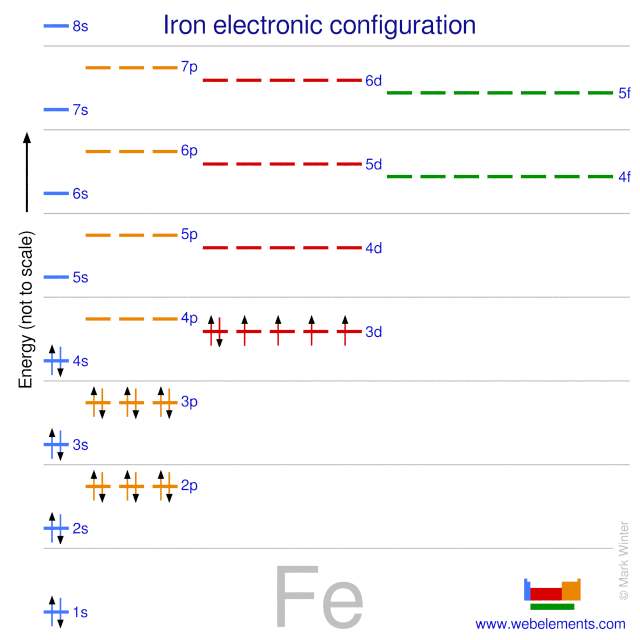
. The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons. Electronic configuration of Fe with 26 electrons is 1S2 2S2 2P6 3S2 3P6. The ground state electronic configuration of Fe3 is.
In this case the valence electrons of iron are eight. The next six electrons will go in the 2p orbital. The atomic number of iron fe is 26.
The electron configuration of the chemical element describes the ground state ie. Which means that Fe3 has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s0 3d5. The ground state describes the lowest possible energy that an atom can have.
Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. The first excited states seems to be mathrmAr4s1 3d7 but isnt the angular momentum not conserved because l0 for mathrms and l2 for mathrmd. The given ground state electron configuration can be written as.
H He Li Be B C 1s1 1s2 1s22s1 1s22s2 1s22s22p1 1s22s22p2 We have a problem. Since Fe originally starts off with the 3d6 4s2 when removing the e- they will come from the outermost shell which is 4s in this scenario. Ar 4s 1 3d 5.
The orbital approximation allows us to express the electronic structure of an atom by reporting its configuration the list of occupied orbitals. Use the table I showed you to figure out the electron configuration. Electron Configuration and Oxidation States of Iron.
Electronic configuration of the element Fe is. Ground state electron configuration of iron Fe is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. 1s2 2s2 2p6 3s2 3p6 4s2 3d6 What is one possible excited state electron configuration for Iron Fe.
The ground state electron config of ___ is Ar4s13d5. In the ground-state electron configuration of Fe3 5 unpaired electrons are. Electron configuration of Fe.
This means there are 26 electrons in Fe at ground state. The ground state electron configuration for a neutral atom of. Question 17 5 points The ground state electron configuration of Fe is 1s22s22p63s23p64s2 1s22s22p63s23p64s24d6 1s22s22p63s23p63d64s2 1s22s23s23p63d6 1s22s23s23p10.
There are 118 elements in the periodic table. The ground state electron configuration of the element ___ is Kr5s14d5. What are the configurations of its first and third excited states.
Electron configuration of Fe is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. The atomic number of Fe is 26 which means that its atoms contain 26 protons in their nuclei and if neutral 26 electrons in their electron clouds. 4s 2 and the term symbol is 5 D 4.
What is the ground state electron configuration for Fe2 The electron configuration for Fe2 will be 1s2 2s2 2p6 3s2 3p6 4s2 3d4 because it has lost two electrons. The periodic table is a tabular. Under this condition today it is well known that the correct electron configuration should be given by this correct image of Iron including the following electron configuration.
1S2 2S2 2P6 3S2 3P6 4S2 3D6. Thats 26 electrons but it. Ar3d 6 4s 2.
The ground state electron configuration of ground state gaseous neutral iron is Ar. The next six electrons will go in the 2p orbital. 1s22s22p63s23p63d64s2 If you look at the Aufbau diagram you can see that the 4s sublevel fills before the 3d sublevel because it has lower.
So you thought wrong. Ground state electron configuration of Fe is. The electron configuration of Fe in its ground state is.
The ground state electron configuration of Fe is. The electron configuration of the ground state of ceFe is mathrm1s2 2s2 2p6 3s2 3p6 4s2 3d6. Ar 3d5 In this case you can only have a maximum of 5 unpaired electrons.
Basically since the electron configuration of Fe is Ar 3d6 4s2 it is important to start by looking at this first rather than looking straight at Chromium which has an electron configuration of Ar 3d5 4s1. In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. The ground state electronic configuration of Fe is 1s22s22p63s23p63d64s2.
There are two types of iron ions. 1s2 2s2 2p6 3s2 3p6 4s2 3d6. What is the ground state electron configuration for Iron Fe.
The element that has a valence config of 4s1 is ___ K. GROUND STATE CONFIGURATION for first 6 elements. 1s 2 2s 2 2p x2 2p y2 2p z2 3s 2 3p x2 3p y2 3p z2 3d 6.
The chemical environment of the Fe3 will ultimately determine whether you have 5 unpaired. The ground state electronic configuration of cobalt Co Ar 3d 7 4s 2 The ground state electronic configuration of iron Fe Ar3d 6 4s 2 The ground state electronic configuration of Nickel Ni Ar 3d 8 4s 2 The ground state electron configuration of copper Cu Ar 3d 10 4s 1. Fe3 ion has 26-323 electrons.
Chemistry questions and answers. Electron as occupying its own orbital. Electron configuration of Iron is Ar 3d6 4s2.
What is difference between ground state and excited state. When 3 electrons are removed to form Fe3 the electronic configuration is. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structureThe chemical symbol for Iron is Fe.
The p orbital can hold up to six electrons. Possible oxidation states are 23.

Which Element Has The Following Ground State Electron Configuration In 2022 Electron Configuration Electrons Configuration
Comments
Post a Comment